Abstract
Objective: Gemtuzumab ozogamicin (GO), the first antibody-drug conjugate (ADC), has attracted the interest of hematologists because over 90% of AML express its target, CD33. Paradoxically, to exert its cytotoxicity, GO is dependent on the cellular function of leukemic cells, including CD33 expression, internalization of CD33-GO complex, and digestion of the acid-labile linker between CD33 antibody and calicheamicin in acidic lysosomes. GO was voluntarily withdrawn from the US market following poor results in the Phase III SWOG S0601 trial. However, we speculate that this result was due to inhibition of the function of these leukemic cells by the chemotherapeutic agents given in combination with GO, hindering its efficacy. Accordingly, to better demonstrate the essential efficacy of GO, we considered that it would be better to enhance the cellular function of leukemic cells than to combine GO with cytotoxic agents. We previously reported that a low, non-toxic dosage of decitabine enhanced GO's cytotoxicity (Kurimoto et al, Leukemia 2013). Based on this result, another group conducted a Phase II trial and revealed significant benefit in a subset of patients with AML (Naval Daver et al, Leukemia 2016). Although GO and subsequent current ADCs depend on lysosomes for activation, lysosome number and activity in tumor cells has not been well elucidated, especially in AML blasts.
Here, for the first time, we report that modulation of the lysosomal activity of leukemic cells enhanced the cytotoxicity of GO. We utilized autophagy to enhance lysosomal activity because autophagy consists of autophagosome formation followed by digestion in the acidic lysosome environment. mTOR kinase is known to inhibit autophagy. An mTORC1/2 kinase inhibitor, PP242, was reported to activate lysosomal function (Jing Zhou, et al. Cell Research, 2013) rather than induce severe cell death at low concentrations and was therefore speculated to increase free calicheamicin by improving the efficiency of linker hydrolysis. Thus, in this study, we examined whether PP242-induced inhibition of mTORC1/2 kinase potentiates the cytotoxicity of GO in AML cells and sought to investigate the mechanism by which such enhancement occurs.
Methods: Human AML cell lines U937, THP-1, HL-60, SKM-1, SKNO-1, MARIMO and KO52 were used. Apoptosis assay and cell cycle analysis was performed by flow cytometry. Lysosomal compartments were labeled with Lysotracker Red DND-99 and fluorescence images were captured by confocal microscopy. The apoptosis mechanism was analyzed by Western blotting.
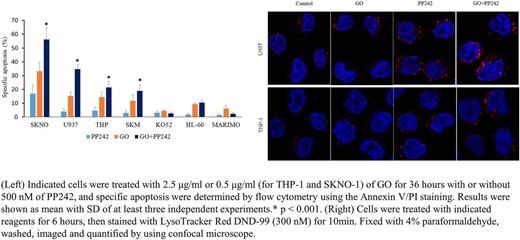
Results: Cytotoxic effect of GO was enhanced by concurrent treatment with a non-toxic concentration (500 nM) of PP242 in U937 (specific apoptosis 15.3% (GO) vs 34.6% (GO+PP242), respectively, P < 0.001), THP-1 (14.5% vs 21.5%, P < 0.001), SKM-1 (11.7% vs 18.9%, P < 0.001) and SKNO-1 (33.3% vs 56.1%, P < 0.001) cells. In contrast, no combinatory effect was detected in HL-60, MARIMO or KO52 cells (Figure, left panel). Next, we used Lysotracker to label and quantify acidic compartments (lysosomes) in U937, THP-1, MARIMO and KO52 cells. The basal Lysotracker fluorescence was weak in all tested AML cell lines. Fluorescence was dramatically increased by the administration of PP242 and the intensified fluorescence was retained in PP242+GO in both U937 and THP-1 cells, as expected (Figure, right panel). However, we observed no significant increase in Lysotracker fluorescence by PP242 in MARIMO or KO52 cells, consistent with the lack of combinatory cytotoxicity. Moreover, PP242 suppressed GO-induced Chk1 activation and G2/M cell cycle arrest, which was considered to be another mechanism of the enhancement of cytotoxicity.
Conclusions: Inhibition of mTORC1/2 kinase by PP242 enhanced the cytotoxicity of GO by increasing lysosomal compartments and promoting the cell cycle by suppressing GO-induced Chk1 activation. Such combination may represent an attractive new anti-leukemia therapeutic strategy. Further, it may help pave the way for the new application of mTORC1/2 kinase inhibitors in effective targeted drug delivery. This use may also benefit the use of other ADCs via utilization of its intrinsic mechanism of action, rather than by the simple combination of strongly cytotoxic drugs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal